Carbon dioxide equivalent (CDE) and Equivalent carbon dioxide (CO2e) are two related but distinct measures for describing how much global warming a given type and amount of greenhouse gas may cause, using the functionally equivalent amount or concentration of carbon dioxide (CO2) as the reference.
Contents
[hide]Global warming potential[edit]
Main article: Global warming potential
Carbon dioxide equivalency is a quantity that describes, for a given mixture and amount of greenhouse gas, the amount of CO2 that would have the same global warming potential (GWP), when measured over a specified timescale (generally, 100 years). Carbon dioxide equivalency thus reflects the time-integrated radiative forcing of a quantity of emissions or rate of greenhouse gas emission—a flow into the atmosphere—rather than the instantaneous value of the radiative forcing of the stock(concentration) of greenhouse gases in the atmosphere described by CO2e.
The carbon dioxide equivalency for a gas is obtained by multiplying the mass and the GWP of the gas. The following units are commonly used:
- By the UN climate change panel IPCC: n×1012 metric tonnes of CO2 equivalent (GtCO2eq).
- In industry: million metric tonnes of carbon dioxide equivalents (MMTCDE).
- For vehicles: g of carbon dioxide equivalents / km (gCDE/km).
For example, the GWP for methane over 100 years is 25 and for nitrous oxide 298. This means that emissions of 1 million metric tonnes of methane and nitrous oxide respectively is equivalent to emissions of 25 and 298 million metric tonnes of carbon dioxide.[1]
Equivalent carbon dioxide[edit]
Equivalent CO2 (CO2e) is the concentration of CO2 that would cause the same level of radiative forcingas a given type and concentration of greenhouse gas. Examples of such greenhouse gases aremethane, perfluorocarbons, and nitrous oxide. CO2e is expressed as parts per million by volume, ppmv.
- CO2e calculation examples:
- The radiative forcing for pure CO2 is approximated by
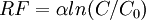 where C is the present concentration,
where C is the present concentration,  is a constant, 5.35 and
is a constant, 5.35 and  the pre-industrial concentration, 278 ppm. Hence the value of CO2e for an arbitrary gas mixture with a known radiative forcing is given by
the pre-industrial concentration, 278 ppm. Hence the value of CO2e for an arbitrary gas mixture with a known radiative forcing is given by 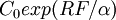 in ppmv.
in ppmv. - To calculate the radiative forcing for a 1998 gas mixture, IPCC 2001 gives the radiative forcing (relative to 1750) of various gases as: CO2=1.46 (corresponding to a concentration of 365 ppmv), CH4=0.48, N2O=0.15 and other minor gases =0.01 W/m2. The sum of these is 2.10 W/m2. Inserting this to the above formula, we obtain CO2e = 412 ppmv.
- To calculate the CO2e of the additional radiative forcing calculated from the 2012 averaged data:[2] ∑ RF(GHGs) = 3.234, thus CO2e = 278 e3.234/5.35 ppmv = 508.8 ppmv
- The radiative forcing for pure CO2 is approximated by
No comments:
Post a Comment